Back to top
Nucleobase Modifications (for DNA)
| Nucleobase Modifications | Position | ||
| 5’ | 3’ | int | |
| 2’-Deoxyinosine (INO) | X | X | |
| 2-Aminopurine | X | ||
| 5-Me-dC | X | ||
| Deoxyuridine | X | ||
| Mixed Bases | X | ||
| Propynyl-dC | X | ||
| Propynyl-dU | X | ||
| TIPS-Alkyne-(C8)-dT | O | O | O |
| TMS-Alkyne-(C8)-dT | O | O | O |
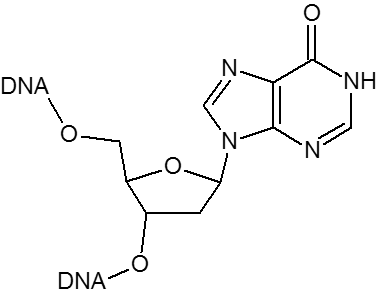
Historically, the first universal base employed was 2’-deoxyInosine (dI). DeoxyInosine is a naturally occurring base that, while not truly universal, is less destabilizing than mismatches involving the four standard bases. Hydrogen bond interactions between dI and dA, dG, dC and dT are weak and unequal, with the result that some base-pairing bias does exist with dI:dC > dI:dA > dI:dG > dI:dT. When present in a DNA template, deoxyInosine preferentially directs incorporation of dC in the growing nascent strand by DNA polymerase.
|
Position
|
Synthesis Scale [µmol]
|
Purification
|
|||||
|
|
0.04
|
0.2
|
1.0
|
15
|
Des
|
HPLC
|
PAGE
|
| 3' |
X
|
X
|
O |
X
|
X | ||
| Int |
X
|
X
|
O |
X
|
X | ||
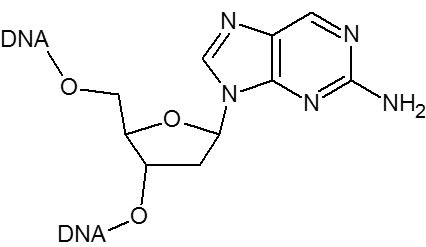
2-Aminopurine can substitute for dA in an oligonucleotide. It is a naturally fluorescent base that is sensitive to the local environment making it a useful probe for monitoring the structure and dynamics of DNA hairpins and for detecting the base stacking state of a duplex. 2-Aminopurine can be destabilizing and slightly lower the Tm.
|
Position
|
Synthesis Scale [µmol]
|
Purification
|
|||||
|
|
0.04
|
0.2
|
1.0
|
15
|
Des
|
HPLC
|
PAGE
|
| Int |
X
|
X
|
O |
X
|
X | ||
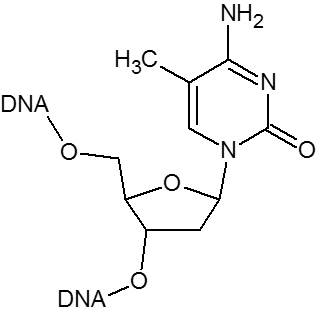
5-Methyl-2'-deoxycytidine when substituted for dC will increase the Tm by as much as 0.5°C per insertion. In addition, the presence of 5-Methyl dC in CpG motifs can prevent or limit unwanted immune responses that otherwise occur if oligos are administered in vivo, which is of particular importance in antisense applications.
|
Position
|
Synthesis Scale [µmol]
|
Purification
|
|||||
|
|
0.04
|
0.2
|
1.0
|
15
|
Des
|
HPLC
|
PAGE
|
| Int |
X
|
X
|
O |
X
|
X | ||
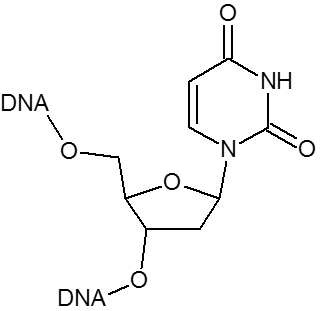
DNA analogue of RNA-U.
|
Position
|
Synthesis Scale [µmol]
|
Purification
|
|||||
|
|
0.04
|
0.2
|
1.0
|
15
|
Des
|
HPLC
|
PAGE
|
| Int |
X
|
X
|
O |
X
|
X | ||
|
Position
|
Synthesis Scale [µmol]
|
Purification
|
|||||
|
|
0.04
|
0.2
|
1.0
|
15
|
Des
|
HPLC
|
PAGE
|
| Int |
X
|
X
|
O |
X
|
X | ||
Substitution of propynyl-dC (pdC) for dC and C-5 propynyl-dU (pdU) for dT are effective strategies to enhance base pairing. Using these base substitutions, duplex stability and melting temperatures are raised by the following amounts: propynyl-dC 2.8°C per substitution; propynyl-dU 1.7°C per substitution.
|
Position
|
Synthesis Scale [µmol]
|
Purification
|
|||||
|
|
0.04
|
0.2
|
1.0
|
15
|
Des
|
HPLC
|
PAGE
|
| Int |
X
|
X
|
O |
X
|
X | ||
Substitution of propynyl-dC (pdC) for dC and C-5 propynyl-dU (pdU) for dT are effective strategies to enhance base pairing. Using these base substitutions, duplex stability and melting temperatures are raised by the following amounts: propynyl-dC 2.8°C per substitution; propynyl-dU 1.7°C per substitution.
|
Position
|
Synthesis Scale [µmol]
|
Purification
|
|||||
|
|
0.04
|
0.2
|
1.0
|
15
|
Des
|
HPLC
|
PAGE
|
| Int |
X
|
X
|
O |
X
|
X | ||
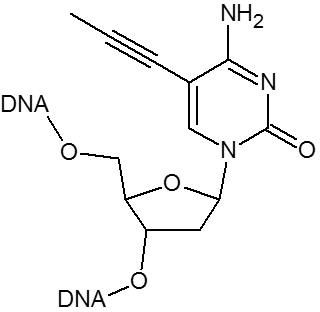
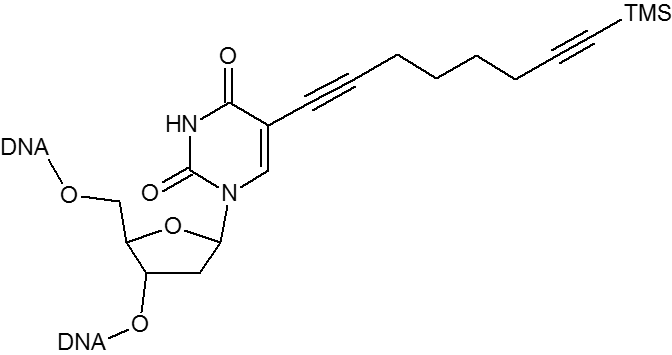
Click chemistry.
|
Position
|
Synthesis Scale [µmol]
|
Purification
|
|||||
|
|
0.04
|
0.2
|
1.0
|
15
|
Des
|
HPLC
|
PAGE
|
| Int | O | O | O | O | O | ||
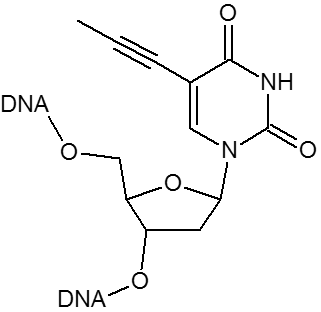
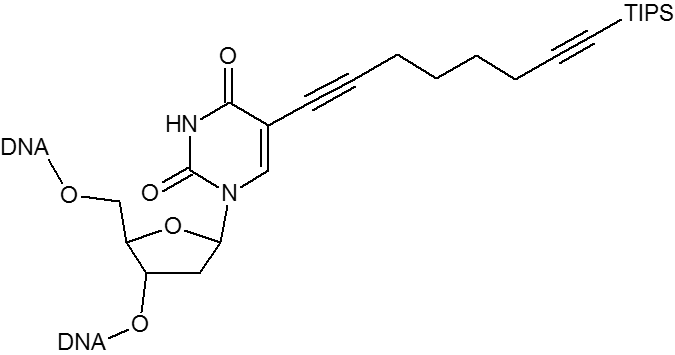
Click chemistry.
|
Position
|
Synthesis Scale [µmol]
|
Purification
|
|||||
|
|
0.04
|
0.2
|
1.0
|
15
|
Des
|
HPLC
|
PAGE
|
| Int | O | O | O | O | O | ||